ARE STYRENIC DERIVATIVES SOON TO BE BANNED FROM OUR PACKAGING? THE “CLIMATE AND RESILIENCE” LAW LAUNCHES THE MOVEMENT…
21 July 2023
What is the cosmetics industry’s responsibility with regards to the application of the Nagoya protocol?
22 November 2023A number of major changes have occurred in the cosmetics and perfumes regulatory landscape during the summer of 2023. Let’s take a look at TWO major new regulatory texts that will have an impact on how you put your next cosmetics projects onto the market.
Allergens: new labelling regulations
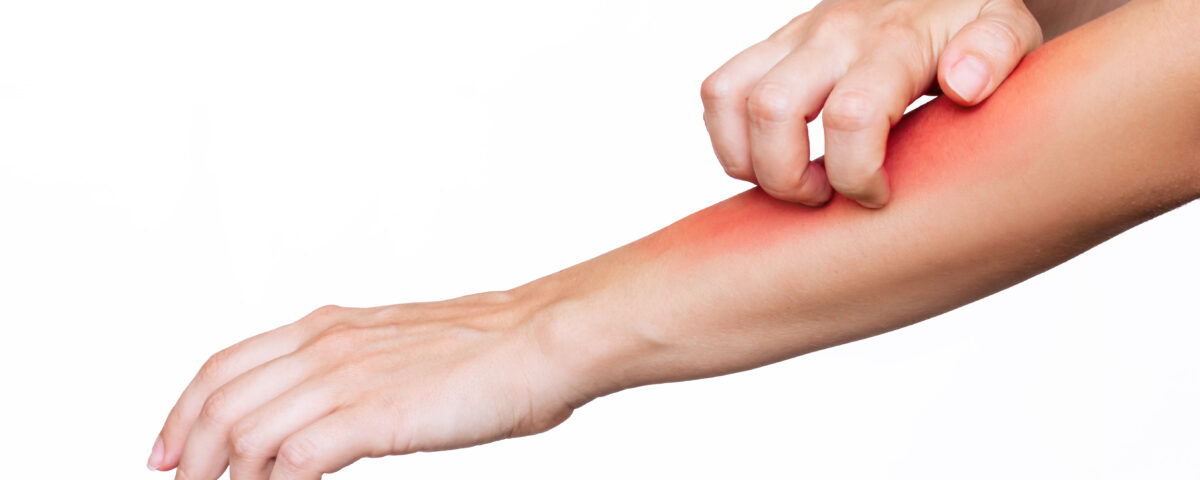
The European (EU regulation 2023/1545) as regards labelling of fragrance allergens in cosmetic products was published in the EU Official Journal on Thursday 27 July 2023; it considerably lengthens the list of allergens that must be included on product labels. As you know, until now, only 24 substances were recognised as potential allergens for humans requiring inclusion on product labels when present over specific content thresholds (0.01% for rinse-off products and 0.001% for no rinse products). This new regulation amends appendix III of EU regulation 1223/2009:
- it adds 45 new lines
- and updates a further 17 lines
Consequently 81 allergens must now be included on labels. The labelling thresholds remain unchanged.
This regulation introduces a further new element: “grouping names”. This means that, labelling can now be simplified for certain substances where they come from the perfume or are added as such, these can now be grouped under the same name.
As regards application deadlines, the regulation states:
- 31 July 2026: all products present on the market must indicate the presence of these new substances on their ingredient lists.
- 31 July 2028: all products which do not respect these new regulations must be taken off the market.
This extension to the list of allergens that must be labelled will involve significant extra work. Even if the 2026 and 2028 deadlines seem far off, CLEAR strongly recommends: ANTICIPATION!
Publication of the IFRA 51st Amendment
In addition to these new elements concerning allergens, the IFRA also published its 51st amendment on 30 June. This new amendment introduces 49 new standards and updates a further 11 existing ones.
This amendment introduces a different approach concerning phototoxic ingredients. Certain phototoxic substances such as Methyl N-methylanthranilate (MNMA) are now subject to restrictions in rinse-off products of certain categories (7A, 9 and 10A). In addition to this category 6 products are now classed as rinse-off products for phototoxicity (before they were considered as no rinse products).
Application deadlines will depend on the concentrate’s production date and the presence of ingredients restricted by IFRA 51:
- 30 August 2023: concentrates produced after notification of the 51st amendment which contain a future prohibited ingredient must be IFRA 51 compliant.
- 30 March 2024: concentrates produced after notification of the 51st amendment not containing prohibited ingredients but which may contain restricted ingredients must be IFRA 51 compliant.
- 30 July 2024: concentrates produced before notification of the 51st amendment which contain a future prohibited ingredient must be IFRA 51 compliant.